The global pandemic has taken a toll on front-line healthcare workers perhaps more than most other occupations. According to a survey by Nursing Standard, 8 in 10 nurses reported their mental health has been affected by COVID-19, and that their chief concerns are worrying about patients, the possibility of infecting family members, and the financial impact of the pandemic.
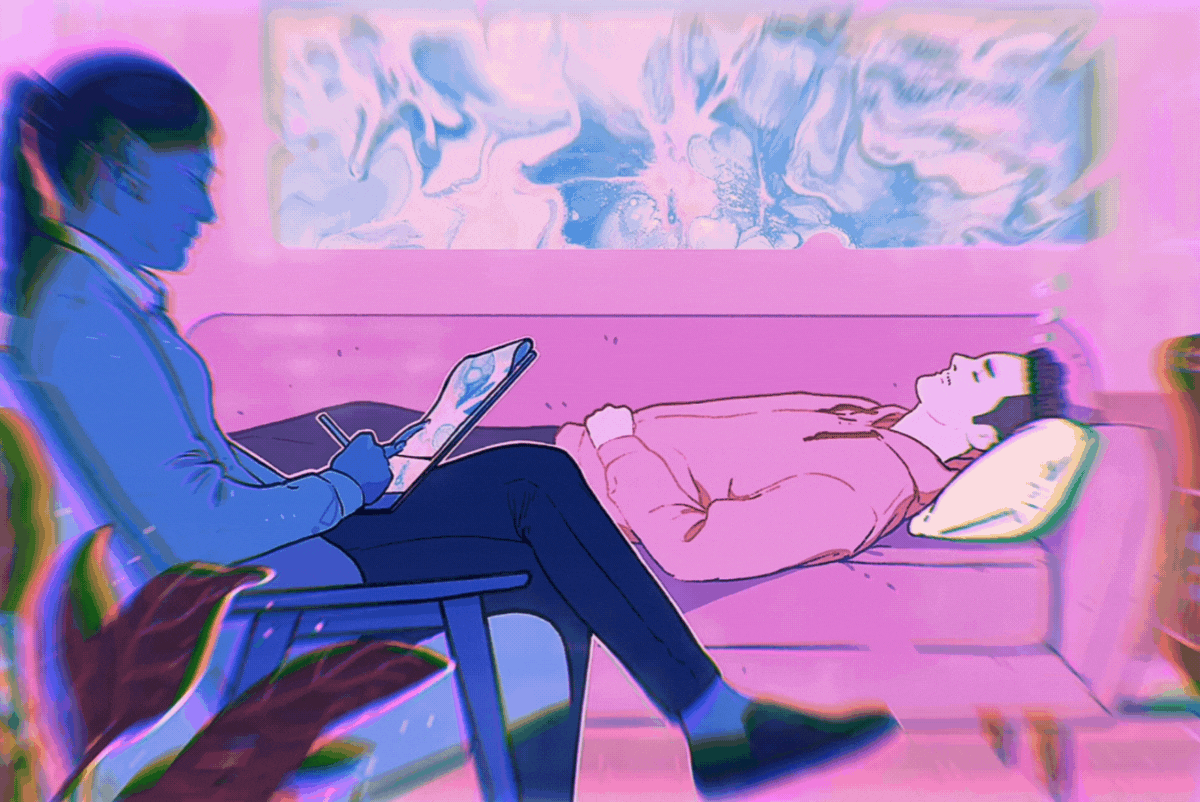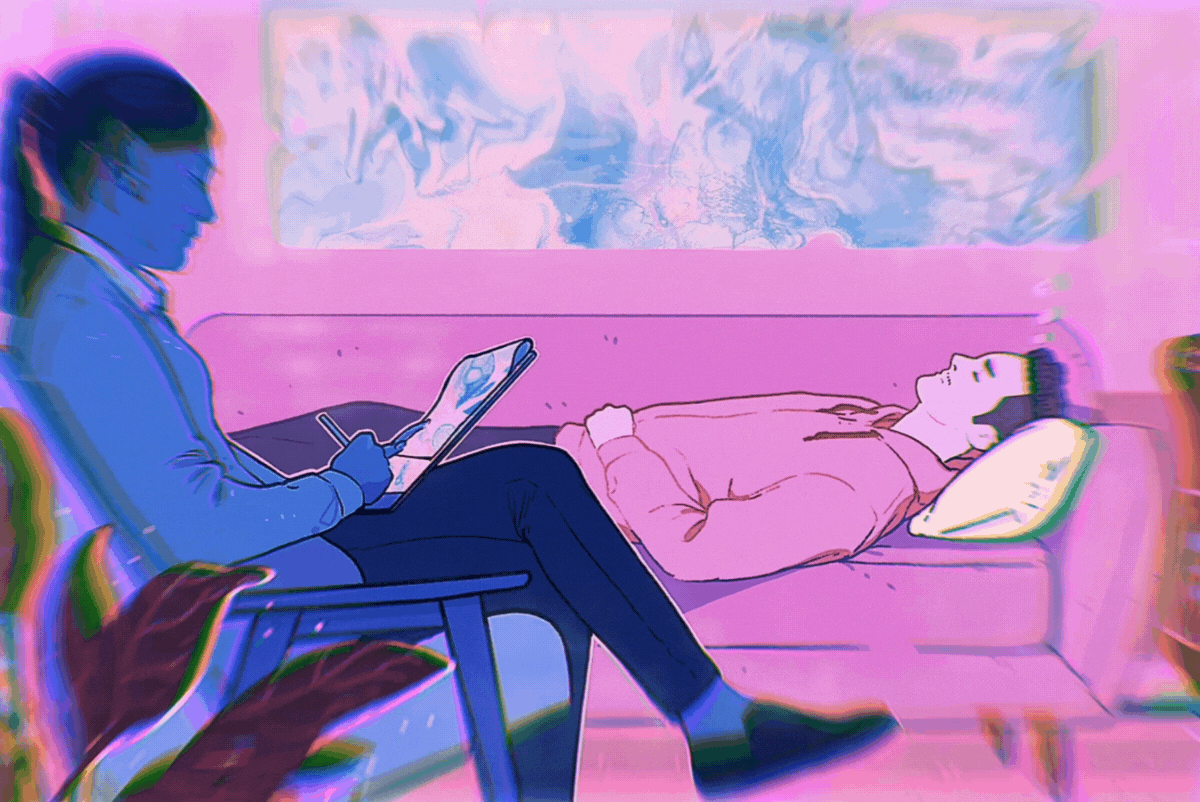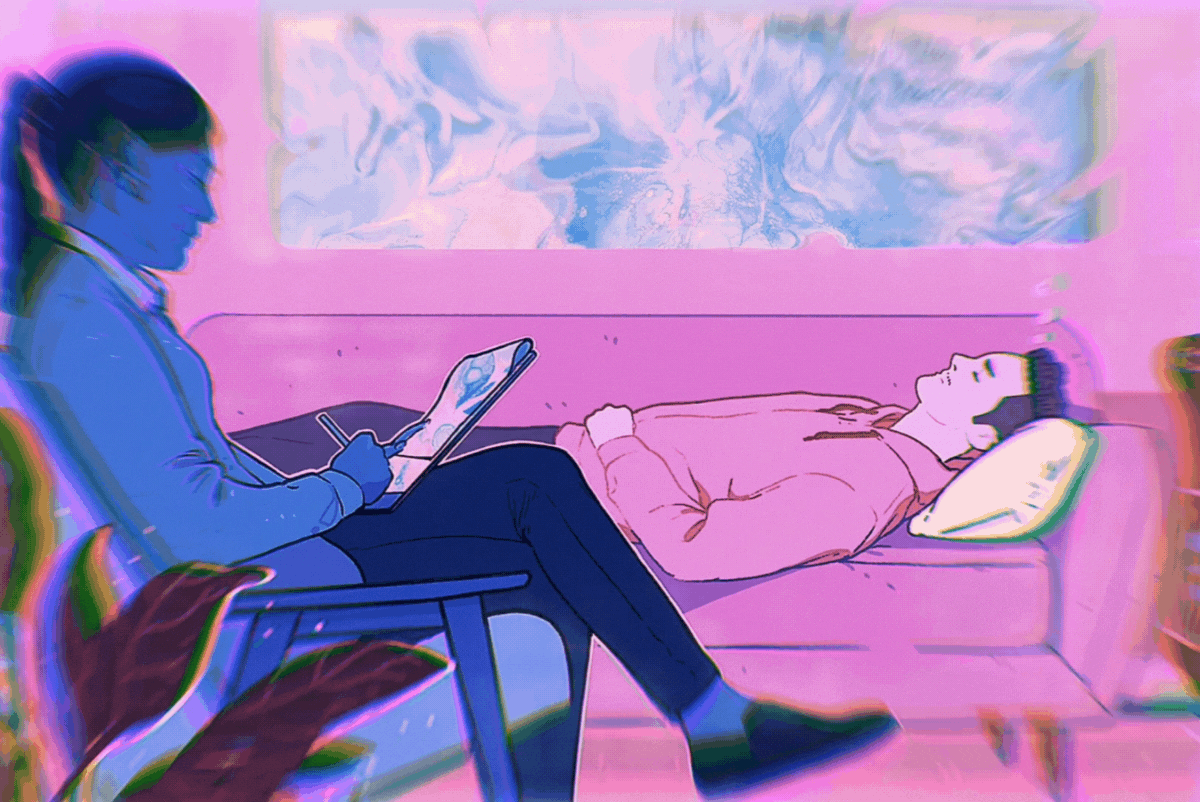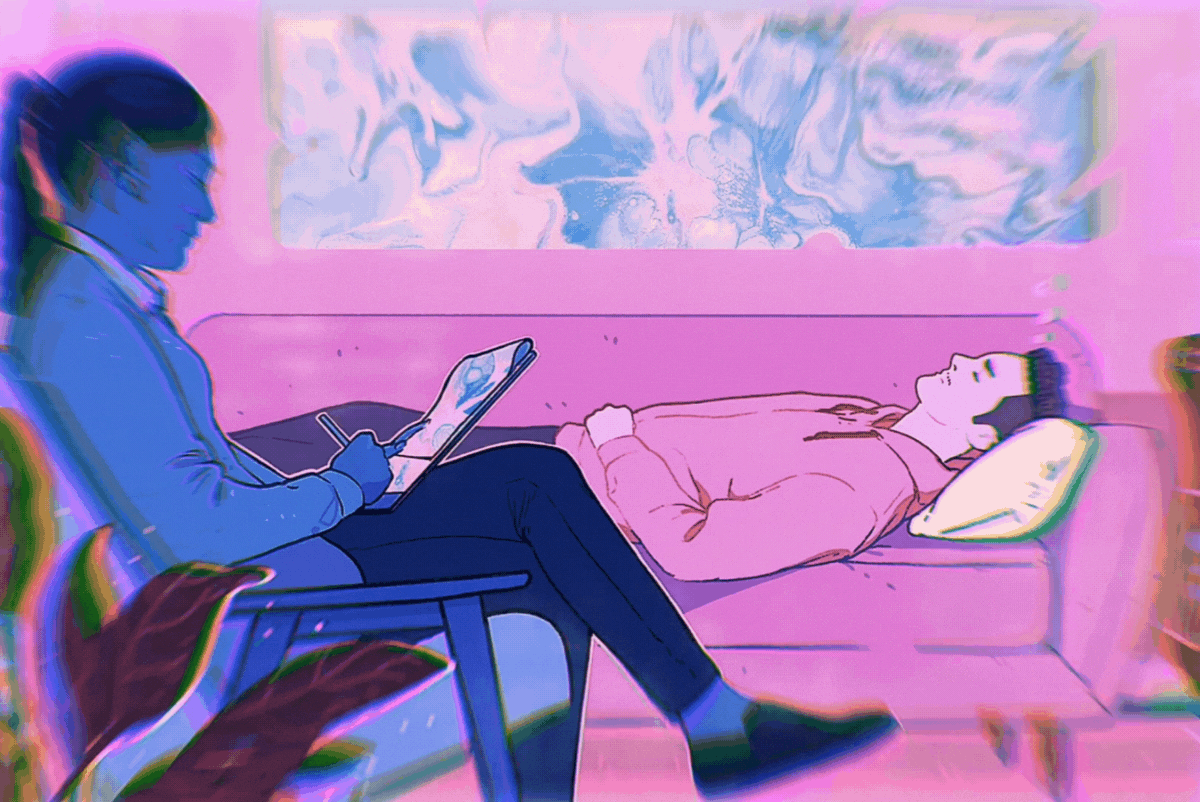
A new clinical trial conducted by biotech company Cybin Inc. and the University of Washington, will use the psychedelic medicine psilocybin to treat frontline clinicians suffering from COVID-related burnout, depression, anxiety, and post-traumatic stress disorder (PTSD). Numerous studies have shown psychedelics such as psilocybin and MDMA effective at treating patients suffering from similar mental health conditions.
The clinical trial involving psilocybin-assisted psychotherapy exclusively for healthcare workers emotionally affected by COVID-19, will be led by Dr. Anthony Back, a board-certified physician at the University of Washington. The study will be held in Seattle, a city hit particularly hard early in the pandemic.
“There is tremendous potential in a collaboration between the University of Washington and Cybin to move the field forward, and this project is an incredibly valuable initial step towards a productive future,” Dr. Back said.
To support the initiative, Cybin developed EMBARK, as a transdiagnostic psychotherapy model that can be adapted to address a range of clinical indications and populations.
“Our nation’s doctors, nurses and clinicians have been shouldering the burden of COVID-19 by taking care of the sickest among us. They’re experiencing high levels of anxiety, depression and burnout. Now it’s our turn to help them,” said Dr. Alex Belser, Cybin’s Chief Clinical Officer. “We are sponsoring research to see if psychedelic medicine, when used with EMBARK’s supportive therapy, can help clinicians recover from COVID-related distress.”