On Oct 31, 2023, the alternative medicine community woke up to some unprecedented positive data demonstrating psilocybin’s safety and efficacy in treating depression.
Cybin, a biotech company working on next-generation psychedelic treatments, announced remarkable results from its Phase 2 clinical trial using a proprietary version of deuterated psilocybin for the treatment of depression. CYB003 is a next-generation psilocybin designed to provide a faster onset of action and to reduce the duration of effects, ultimately thinning the financial barrier to access of this medicine in a medical setting.
The trial’s interim data demonstrated “rapid, robust, and clinically significant reduction of depression symptoms” three weeks after a single 12mg dose of CYB003.
More specifically, three weeks after a single administration of the drug, 53.3% of patients showed at least 50% reduction in depressive symptoms (≥50% reduction in MADRS), and 20% of participants entered remission, meaning they no longer met the clinical definition of depression.
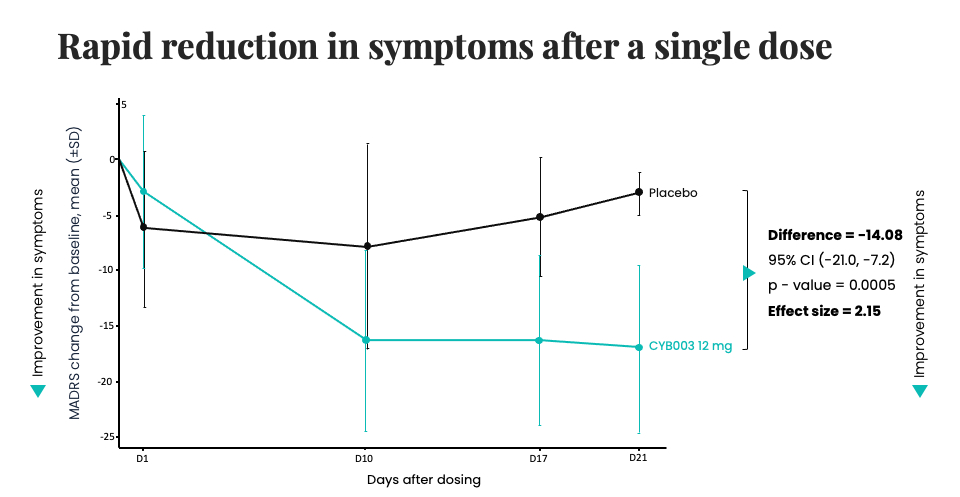
The results demonstrate a 14-point Montgomery-Asberg Depression Rating Scale (“MADRS”) score reduction post-CYB003 administration compared to a 1.82-point for traditional antidepressants compared to placebo. The stark difference in efficacy is what makes the results truly unprecedented.
We should not dismiss the speed of these results. Typically, significant reductions in depression symptoms take longer than three weeks, making CYB003’s onset of action faster than conventional treatments, which can take more than several weeks to simply show any effect at all.
Provided Cybin is able to replicate these results in its Phase 3 trials, we may see another depression solution in the market in the next few years.
“The overwhelmingly positive interim results for the 12mg dose of CYB003 are extremely encouraging for patients and providers. The efficacy demonstrated at that dosage showed an unprecedented reduction in depressive symptoms compared to currently available treatments,” said Doug Drysdale, Chief Executive Officer of Cybin. “With these encouraging results in hand, we look forward to sharing the full complement of topline data later this quarter, and 12-week durability data in the first quarter of 2024. Our planning continues as we prepare for a larger international, multisite Phase 3 trial in early 2024 to further evaluate the safety and efficacy of CYB003 in people suffering from MDD.”
Cybin isn’t the only company advancing through clinical trials. Earlier this year, Compass Pathways began recruiting for the largest Phase 3 clinical trial investigating the safety, efficacy and tolerability of psilocybin for treatment-resistant depression (TRD), totaling 946 participants, and is expected to complete the study by 2025. If Compass delivers positive data, we may see FDA approval for psilocybin therapy in late 2025.