In the paper titled Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors, Vargas et al. discovered that the activation of intracellular 5-HT2A receptors through psychedelics may be responsible in their promoting of neuronal plasticity and antidepressant-like effects.
It has long been pondered exactly how psychedelics cause antidepressant effects. Past research has established a clear association between psychedelics and the role of membrane-bound 5-HT2A receptors in their mechanism of action. In addition, the use of psychedelics has been shown to promote neuronal plasticity which may be attributed to their antidepressant-like effects.
But this paper finally gives light to a new facet of the mechanism of action of psychedelics: it may not be membrane receptors that are responsible for the antidepressant effects of psychedelics, but rather receptors found inside the cell. Through a series of experiments, this team of researchers have found conclusive evidence that intracellular receptors directly affect the neuronal growth in the form of dendritogenesis and spinogenesis.
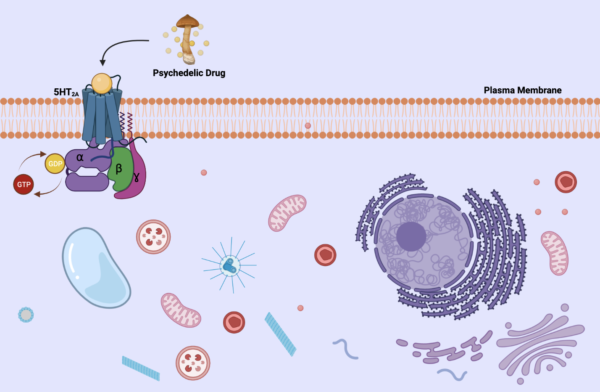
To better understand this, a quick refresh of basic biology would be helpful. There are many different types of cells in our bodies, but they all have a cell membrane that houses organelles inside. In some cell membranes (also called plasma membranes or lipid bilayers) there are receptors lodged in them that help extracellular molecules pass messages to the inside of the cell. In Figure 1, here is a basic diagram of an extracellular 5-HT2A receptor. This receptor is part of a family called serotonin receptors, which, as its name states, bind to serotonin. When a psychedelic is introduced in the body, they bind to this receptor, which has been predicted that their hallucinogenic effects are brought on due to this activation.
Understanding how lipophilicity affects drug action
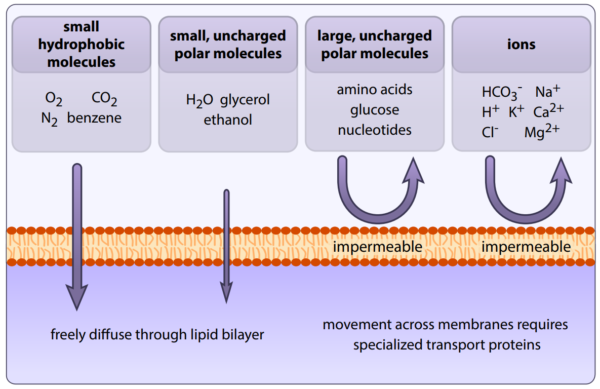
Since the plasma membrane is a lipid bilayer, only lipophilic molecules can pass through, meaning only molecules that have an affinity for fats/lipids can easily cross via passive diffusion. Anything that is lipophobic is unable to cross through the membrane, thus requiring some sort of transport system, such as protein pumps or channels.
In this paper, researchers noticed that more lipophilic agonists (a chemical that activates a receptor) exhibited greater abilities to promote neuronal plasticity than their lipophobic counterparts. This led them to hypothesize that if lipophilic molecules are able to increase plasticity, then that means they are crossing the membrane and binding to an intracellular pool of 5-HT2A receptors that are responsible for psychedelic-induced neuronal growth.
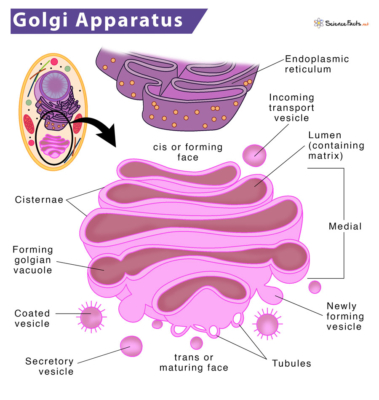
Researchers performed experiments to determine where exactly these receptors might be, since the inside of a cell is vast. By performing live cell imaging and immunocytochemistry experiments, researchers that there was high localization of 5-HT2A receptors in the Golgi apparatus of neurons, which aids in the processing and sorting of proteins.
Testing drugs of different lipophilicities
With researchers knowing where inside the cell there was a greater expression of 5-HT2A receptors, they then wanted to determine whether activating these receptors was essential for psychedelics’ ability to promote neuroplasticity.
To do this, they took three permeable, lipophilic molecules (DMT, psilocin, and ketanserin) and modified them to be unable to pass through the plasma membrane by making them highly charged. DMT and psilocin are agonists of the 5-HT2A receptor, while ketanserin is an antagonist, meaning it stops the action or effect of it.
Having two groups of lipophilic and lipophobic molecules now allowed them to determine whether these drugs had an effect on intracellular response. Researchers used a process called electroporation to force otherwise impermeable molecules through the plasma membrane.
They introduced 1µM of each drug from both groups to freshly dissected rat embryonic neurons and saw that DMT and psilocin both promoted the growth of new dendrites (tree-like appendages in neurons) regardless of electroporation.
But the highly charged molecules like N,N,N-DMT and psilocybin only promoted neuronal growth when electroporated, meaning only internal receptors were at play. This was a key part of the experiment, since it proved that only permeable 5-HT2A agonists increased neuronal growth.
Comparing results with serotonin
Now, if psychedelics caused neuronal growth by the activation of intracellular receptors, then, theoretically, so should serotonin. To test this, researchers repeated the electroporation test with serotonin and found that neuronal growth only occurred when electroporated, substantiating the hypothesis.
Researchers then introduced SERT (a serotonin transporter) into the plasma membrane of neurons to allow the crossing of serotonin into the intracellular space. Only cells with SERT showed neuronal growth, since these transporters allowed serotonin inside. When they introduced citalopram, a SERT inhibitor, they found that this blocked the effects of serotonin. To compare results, researchers used DMT and noted that citalopram did not block its function. Then, when they treated the cells with ketanserin, a 5-HT2A antagonist, neither DMT nor serotonin were able to promote neuronal growth. This proved that DMT and intracellular serotonin promote neuroplasticity by 5-HT2A in vivo.
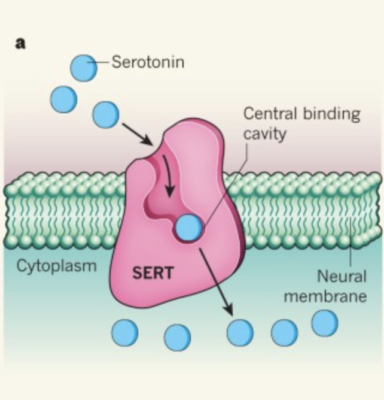
It is possible this is why antidepressants like SSRIs have low success rates. SSRIs increase extracellular concentrations of serotonin, but these molecules cannot cross through the plasma membrane. Considering what this paper has uncovered, it is possible that SSRIs are treating depression incorrectly by promoting an increase of serotonin that is unable to enact any sort of change on neuroplasticity or on any system that would give rise to antidepressant-like effects. If there was a way to get the extracellular concentration of serotonin to go inside neurons, then there may be a possibility that their success rate would significantly increase.
The science behind psychedelics gets clearer
This paper has helped elucidate that a substantial proportion of 5HT2A receptors in cortical neurons are localized in the Golgi apparatus, which means intracellular signaling may be at play. They predict that it is possible that the Golgi protonates (adds protons) psychedelics when ingested and cause their sustained signaling and retention, which in turn results in neuronal growth.
Furthermore, serotonin is unable to cross the plasma membrane without the help of transporters, thus researchers pondered whether serotonin is the endogenous ligand of these intracellular receptors at all. An interesting thing they mentioned is that concentrations of DMT and serotonin in the cortex are comparable, thus is DMT the endogenous ligand for intracellular receptors?
An interesting observation made during the experiment was head-twitch responses (HTR) in SERT(+) mice who were dosed with a selective serotonin-releasing agent. Head-twitch responses are rapid side-to-side head movements that occur in rats and mice after administering serotonergic hallucinogenic drugs; these movements help researchers determine if a drug is hallucinogenic. Mice exhibited HTRs when serotonin was allowed into the intracellular space through SERT, thus researchers ponder whether intracellular 5-HT2A receptors may be responsible for causing the hallucinogenic effects of psychedelics.
But most importantly, this paper implores us to further look at endogenous psychedelics (e.g. DMT) as possibly having a role in health or disease.
Future research can add on to these findings by determining whether intracellular 5HT2A signaling is distinct from membrane signaling. Then, a clearer picture on the mechanism of action of psychedelics can begin to be elucidated.





