
Substance abuse and addiction are pervasive issues that often give rise to debilitating mental disorders. Astonishingly, nearly 1-in-3 adults experienced either a substance use disorder or any mental illness in the past year alone. Additionally, 1-in-10 Americans over the age of 12 have Alcohol Use Disorder and 1.3 million people are dealing with cocaine use disorder according to the NIH.
However, a ray of hope emerges on the horizon with the advent of MEAI, an extraordinary compound that belongs to the category of “Next-Gen” psychedelics. What sets MEAI apart from its psychedelic counterparts is its remarkable ability to remain non-hallucinogenic and non-addictive, ushering in a new era of potential treatments.
Today, we have the privilege of delving into the profound potential that MEAI holds for tackling alcohol and cocaine addiction. Joining us is none other than Professor Mark Haden, an esteemed figure in the field. Professor Haden is the former Executive Director of MAPS Canada (Multidisciplinary Association for Psychedelic Studies), as well as an Adjunct Professor at the prestigious University of British Columbia’s School of Population and Public Health. Notably, Professor Mark Haden has recently embarked on an exciting journey with Clearmind Medicine [NASDAQ: CMND], an innovative biotech company leading the way in developing groundbreaking treatments for binge behavior and mental health disorders. This includes addressing conditions such as alcohol use disorder, binge eating, and depression. Clearmind Medicine owns the patents for MEAI, which is on the cusp of entering human trials with the Food and Drug Administration (FDA), marking a significant milestone in its journey towards potential approval and widespread use.
Maria: What is MEAI? Talk to us about its history and how its anti-addictive properties were discovered. How can MEAI break the addiction cycle?
Mark Haden: MEAI is a new psychedelic compound (5-methoxy- 2-aminoindane) that was first used as a recreational drug — with some irresponsible usership in its early days -initial users saw a potential to prevent binge drinking. In these anecdotal cases, when individuals consumed MEAI with alcohol, they stopped wanting to drink altogether. Clearmind acquired the patents for MEAI and significantly expanded its intellectual property to 6 patent families related to MEAI.
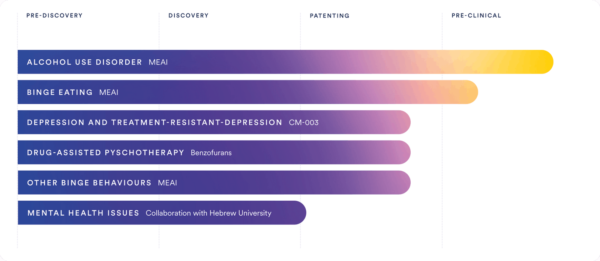
We’re now focused on the safe exploration of the compound as a pharmaceutical treatment for alcoholism, cocaine addiction, and obesity, and are in conversations with regulatory bodies in the US, and Israel, to begin human clinical trials. Clearmind sees the promise in MEAI as potentially innerving neural pathways such as 5-HT1A, which leads to decreased impulsivity and “sensible behavior.” Clearmind’s novel treatment is expected to be non-hallucinogenic and non-addictive.
Maria: So, how does MEAI differ from traditional psychedelic drugs?
Mark: From the anecdotal evidence we’ve gathered, as well as our findings from the preclinical results so far, we have reason to believe that MEAI treatment may offer an effective solution for addictions such as alcohol and cocaine. The compound’s effect seems to function like an “enough switch” in the brain, stopping all further desire for alcohol or cocaine and preventing overuse.
MEAI is an innovative compound, a category nicknamed “Next-Gen” psychedelics, mostly due to the fact that it is not hallucinogenic. MEAI does not offer the “high” typically associated with psychedelics as a kind of spiritual or insightful experience. It is non-hallucinogenic, and delivers the kind of elated and sociable feeling that typically comes after 1-2 drinks. Traditional psychedelic therapy is offered for a very limited number of times (The safety and efficacy of {+/-}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder) and as adjunct to psychotherapy. We believe MEAI’s potential safety profile as detected in research so far indicates a likelihood that it can be used daily, or at any point that an individual might be craving a drink or experiencing an addictive urge and not necessarily in conjunction with psychotherapy.
Maria: Can you describe the MEAI experience? How does it curb your alcohol “appetite” and trigger the satiation response?
Mark: An analogy we often use to describe this is an “enough switch.” It’s like the appetite is suddenly switched off, as regards continued oral consumption of alcohol, cocaine and possibly even food.
Maria: MEAI can be self-administered. Does that mean that if clinical trials go well and the compound is FDA approved, people would be able to get it through prescription?
Mark: In principle this is certainly the target and this is how we design our first in human clinical trial.
Maria: This question is a bit out there, but is there a possibility for MEAI to be sold over the counter similarly to other products designated to curb addiction such as Nicorette for smoking cessation? Would that be safe? If so, then MEAI would be able to be purchased by anyone who needs help to reduce their alcohol consumption and who is not necessarily diagnosed with AUD.
Mark: Indeed this is premature to discuss but Clearmind is developing MEAI as a pharmaceutical pill that offers an appealing approach to healing alcoholism (which is responsible for 3 million deaths annually around the world). Clearmind’s treatment is intended to be delivered in the form of a pill, similar to the new generation of compounds recently approved by FDA to curb overeating. So far the safety profile of MEAI is very promising. We’re still in early stages of research, but we’re seeing positive results in both safety and efficacy, and are examining the “reward potential” of the molecule to ensure it’s non-addictive – and we’re seeing positive results there too. MEAI will be the focus of human trials with the FDA in the US, and we’ll be able to learn much more about how this molecule affects human beings. But from the multiple anecdotal cases we heard as we researched this molecule early on, we’re very optimistic. As studies advance, we hope to learn more about exactly why MEAI seems to be so effective in stopping binge behaviors like alcohol and cocaine addictions.
Maria: So, how does MEAI compare to other treatments in the market?
Mark: The only FDA-approved medications for AUD are Acamprosate, Naltrexone (Vivitrol), and Antabuse (disulfiram). They show relatively low efficacy and bring with them adverse side effects such as vomiting, mood disorder, and seizures. To date, there are no FDA-approved medication treatments for cocaine use disorder or cocaine addiction. Abstinence programs such as 12-Step/AA have estimated success-rates of 5%-10%. Even in psychedelics, only 2% of active or completed clinical trials in the sector relate to alcohol issues.
Maria: I believe Clearmind did some preclinical research on rats to test whether MEAI is safe and whether there is supporting evidence that it has the capacity to curb addiction. Talk to me about this study. Did you test this on alcoholic rats? How does that work? What were the results?
Mark: First, we trained groups of mice to consume alcohol for five weeks. The groups were then given varying daily doses of the new treatment, with intermittent access to alcohol and water. After two weeks, researchers found a significant reduction in alcohol consumption in mice receiving the treatment. The control group showed no statistical significant reduction. Following the behavioral paradigm and to evaluate safety, histopathological testing was conducted on selected organs (heart, lungs, liver, kidneys, brain, pancreas, spleen, and thyroid gland) of tested animals to determine possible toxicological effects of MEAI. No treatment-related histological changes were observed among all MEAI treatment groups compared to control animals.
Maria: MEAI will soon enter human clinical trials with the FDA. When will trials begin?
Mark: We hope to begin human trials in Q2 of this year. This will be a game-changer for our company as well as for the future of psychedelic research. Our first clinical site will be in Israel: the agreement has been signed with the IMCA center in Ramat Gan, Israel. The trial will be led by Prof. Mark Weiser, head of the Psychiatric Division at the Sheba Medical Center, whose specializations include cognitive impairment in persons with psychiatric disorders such as substance abuse, depression and personality disorders. The study was already approved by the IMCA ethics committee (which is equivalent to the US Institutional Review Board, or IRB).
Final Thoughts
While traditional psychedelic therapies are typically limited in their application and often require adjunct psychotherapy, MEAI shows potential for daily use, allowing individuals to address cravings and addictive urges as they arise. MEAI holds immense potential to revolutionize addiction treatment by providing a safe and effective solution for individuals struggling with alcohol and cocaine addiction. The ongoing research and human trials, coupled with the favorable safety profile and promising efficacy, instill optimism for the future of MEAI as a game-changing therapeutic option in the realm of mental health and addiction recovery.
If you’re interested in being part of Clearmind’s clinical trials, you can visit www.clearmindmedicine.com.

![Compass Pathways Q4 Earnings Call Highlights [Is CMPS Closing In On MindMed?]](https://psychedelicspotlight.com/wp-content/uploads/2022/06/maxresdefault-47-600x338.jpeg)



