
A small-scale Phase 1 study using synthetic psilocybin to treat anorexia nervosa (AN) published promising results in Nature Medicine. According to the study, 40% of patients saw a significant reduction in eating disorder psychopathology after three months.
Anorexia nervosa (AN) is a costly mental illness which has no proven treatments that could address the core symptoms. Unfortunately, less than half of patients recover and relapse rates can reach up to 50%. Psilocybin, the main psychoactive ingredient in magic mushrooms, is believed to be a partial agonist of the 5-HT2A serotonin receptor. Researchers believe that psilocybin therapy may hold potential benefits for the treatment of AN due to considerable evidence indicating that people suffering from anorexia nervosa exhibit changes in serotonin activity that may be responsible for AN symptoms. More specifically, it appears that there is a dysfunction of the 5-HT and 5-HT2A receptors in patients with AN. The main thesis is that psilocybin-therapy may be able to treat symptoms of AN because of the way it interacts with the 5-HT2A receptor.
Key Takeaways:
The investigator-initiated open-label study study explored whether a single 25mg dose of COMP360 psilocybin, a synthetic version of psilocybin patented by biotech company Compass Pathways, is safe, efficacious, and tolerable in 10 female patients diagnosed with anorexia nervosa. The main takeaways were:
- 40% of participants saw a clinically significant reduction in eating disorder psychopathology at the three-month follow-up mark.
- Participants saw significant reductions in shape concerns at the one-month follow-up and weight concerns at the three-month follow-up.
- Changes in eating concern were approaching significance at three-month follow-up.
- The body mass index (BMI) increased for five patients at three-month follow-up. However, the changes in BMI were not significant.
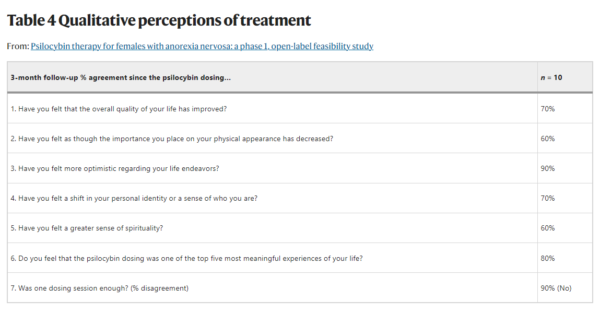
- According to participants the psilocybin experience was meaningful with 90% of participants reporting feeling more positive about life endeavors and 80% reporting that the experience was “one of the top five most meaningful of their life”. Moreover, 70% of participants indicated that they experienced “a shift in personal identity and overall quality of life.”
- The psilocybin treatment was well tolerated with no serious secondary effects. Some mild and short-lived adverse effects were reported such as headache, fatigue and nausea.
Anorexia nervosa is characterized by intense fear of gaining weight. Patients typically have a distorted perception of food and their shape which unlimitedly leads to food restriction. The researchers in the study speculated that since psilocybin therapy has demonstrated to improve symptoms of anxiety and stimulate cognitive flexibility, it may disrupt critical symptoms of AN “including eating disorder (ED)-related preoccupations, rigid thinking styles and entrenched behavioral patterns.” More specifically, the researchers believe that psilocybin’s capacity to improve openness, cause transformative experiences and increase emotional awareness may aid in a re-organizing AN patients’ values by “shifting the relative importance of shape and weight and/or induce greater permeability to new attitudes and behaviors by directly targeting these features.”
There are two interesting parts of the study worth mentioning: one related to the patient responses in the 3-month follow-up survey assessing their psychopathology, and another related to some possible adverse events that may arise in the treatment of patients suffering from AN.
When asked whether one dosing session was enough, 90% of participants indicated that it wasn’t. Seeing that the researchers administered a single 25mg dose of COMP360 psilocybin, it would be interesting to see whether the responses would vary if a second dose was administered, similarly to what the company has decided to test in its largest psilocybin for depression clinical trial.
When it comes to adverse events, although there were not serious adverse reactions to the treatment, there were two cases of hypoglycemia ( low blood sugar) on the dosing day which were resolved within a day. The researchers hypothesize that this was related to a prolonged period of fasting on the dosing day which is common with psilocybin use and was not directly related to the treatment. AN patients often suffer from malnutrition and low levels of carbohydrates which can lead to low blood sugar while in a fasting state. These hypoglycemia occurrences however indicate that future AN research in conjunction with psilocybin should pay attention to blood glucose levels before and after the psilocybin treatment since there are risks associated with hypoglycemia in AN, including sudden death.
People suffering from anorexia nervosa may be eligible for Compass’s Phase 2 is clinical trial, recruiting 60 participants with five trial sites in the US, UK, and Ireland.
Learn more here.




