
In the world of health and medicine, the news seems to be getting trippier by the day. Case in point: a recent development out of North Carolina, where a House committee has approved a bill that could create a $5 million grant program to research the therapeutic potential of psilocybin and MDMA. Meanwhile, a federal agency has announced a whopping $1.5 million in funding to explore psychedelics as a means of treating drug addiction. And in Phoenix (of all places), psychedelic churches have scored some major court victories in their quest for legal status. But perhaps the most mind-bending news of all: L.A. dispensaries are now openly selling ‘magic mushrooms’ as the state considers decriminalization. Brace yourselves, folks: it’s about to get psychedelic up in here.
NC House committee advances bill that supports psychedelic-assisted therapy
A North Carolina House committee has approved a bill to create a $5 million grant program to support research into the therapeutic potential of psilocybin and MDMA and to create a ‘Breakthrough Therapies Research Advisory Board’ to oversee the project.

The measure does not legalize the psychedelics, but it would provide funding for two competitive grants through the state Department of Health and Human Services (HHS) for eligible research initiatives focused on “breakthrough therapies.”
Starting in August 2024, the department would be required to accept grant applications from in-state research entities and academic institutions that can show they’re capable of carrying out the studies, including clinical trials involving adults 21 and older.
The bill, which has now been referred to the House Appropriations Committee before potentially receiving consideration on the floor, says that “the recipient must attest that the grant funds will be used to conduct research in this State on the use of one of two psychedelics, MDMA and psilocybin, and that the research will adhere to all FDA protocols and all applicable federal law.”
The grants are meant to fund three years of research, and recipients would need to submit a report with findings and recommendations to a Breakthrough Therapies Research Advisory Board and health department by January 15, 2028.
The board would be responsible for selecting the two grant recipients and notifying a joint legislative committee about their selection. Each recipient would receive $2.5 million.
Federal agency announces $1.5 million in funding for research on psychedelics to treat drug addiction

A federal health agency is soliciting proposals for a series of research initiatives meant to explore how psychedelics could be used to treat drug addiction, with plans to provide $1.5 million in funding to support relevant studies.
As public interest in the therapeutic potential of psychedelics continues to grow, the National Institute on Drug Abuse (NIDA) this week published three notices of funding opportunities (NOFOs) for research projects to better understand how drugs like psilocybin and ayahuasca could help people with substance use disorders (SUDs).
While all three notices focus on the same overall objective, one would primarily focus on the actual mechanisms of psychedelics, while the others would need to involve clinical trials with human subjects.
“While broad theoretical frameworks for the mechanisms of psychedelic-induced neurobiological and behavioral changes have been recently posited, empirical tests and refinements of these overarching theoretical frameworks are necessary to move the field forward,” NIDA said in a notice for one of the clinical trial opportunities.
NIDA emphasized that, for this type of research, it would be necessary to utilize “modern neuroimaging and behavioral analytic tools” to illustrate the “changes are elicited by psychedelics.”
With that kind of information, “the field would be better equipped both to identify the key neuroplastic adaptations that signal symptom improvements and to design effective future psychedelic therapies.”
NIDA offered examples of questions that it hoped to answer with such research:
- What specific cognitive constructs relevant to SUD treatment (e.g., cognitive control, social/affective processing, predictive/sensory processing, interoception) are modulated by psychedelics?
- What, if any, core changes to neurobiology do these compounds facilitate that account for their wide-ranging clinical potential?
- How do the underlying network connectivity and task evoked activation scale with and/or predict the magnitude and duration of the observed effects of psychedelics?
- What are the roles of psychedelic compounds in altering the dynamics of large-scale brain networks and specific circuits? What is the relationship of these changes to behavior relevant to SUD?
- What are the temporal trajectories of neurobiological, cognitive, and behavioral effects of psychedelics and the relevant dose-response relationships?
- What are the effects of these compounds both within the canonical 5HT2 receptor pathway and on additional signaling pathways (e.g., dopaminergic)?
- Are the neuroplastic effects of psychedelics observed in animal studies replicable in humans? Are these changes responsible for the long-lasting symptom improvements seen in clinical studies?
For the non-clinical trial research opportunity, NIDA said that the “overarching goal” is to “elucidate and validate the molecular, cellular, circuitry and structural mechanisms and pathways that underly the pharmacology of psychedelic compounds for treating substance use disorders (SUDs) and related psychiatric and neurological co-morbidities.”
NIDA said it plans to distribute four awards totaling in $1.5 million in funding collectively in the 2024 Fiscal Year.
Eligible applicants include universities, non-profit organizations, for-profit businesses, state and local governments and federal agencies.
Psychedelic churches in Phoenix score court wins over legal status
Three years after law enforcement agents raided the home of Clay Villanueva — a Phoenix pastor steeped in the psychoactive brew known as ayahuasca — his lawsuit against the Drug Enforcement Administration has been greenlit for trial.
Though he has since passed away, Villanueva’s Vine of Light church was once one of several ayahuasca churches in Arizona who are suing the government for legal status. In recent weeks, two of the churches have scored wins in court as they fight over their legal status.

On May 4, U.S. District Court Judge Roslyn Silver ruled that the case originally brought by Villanueva and a church in Tucson, the Arizona Yage Assembly, can move forward to trial. In March, U.S. District Court Judge Susan Bolton allowed a claim by the Church of the Eagle and the Condor in Phoenix to move forward, rejecting the DEA’s requests to dismiss the case.
While Villanueva’s lawsuit originally alleged a large-scale conspiracy between the DEA and local law enforcement, it now has narrowed in scope to a claim under the Religious Freedom Restoration Act for the AYA. The lawsuit claimed that the DEA’s process for granting a religious exemption for the use of ayahuasca is illegal.
Should the case succeed, the church would be immune to federal prosecution.
L.A. dispensaries openly sell ‘magic mushrooms’ as state weighs decriminalization
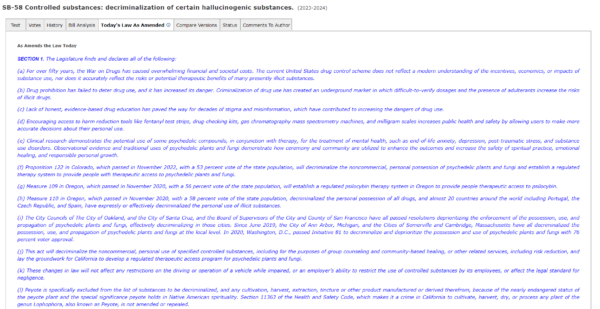
As the state Legislature considers a bill to decriminalize several psychedelics including psilocybin, some L.A.-area businesses are openly selling the potent hallucinogen. Although cannabis is legal statewide, no Southern California municipality or county has followed the lead of Oakland, San Francisco, and Santa Cruz by decriminalizing magic mushrooms.
As evidence of their therapeutic benefits grows and states including Oregon and Colorado legalize or decriminalize magic mushrooms, some Democrats in Sacramento are pushing to make a similar change in California.
Senate Bill 58, currently wending its way through the Legislature, aims to eliminate criminal penalties for possessing, growing and sharing small amounts of several psychedelic substances including psilocybin, ibogaine and DMT.
Sen. Scott Wiener (D-San Francisco) introduced a previous version of the bill last year; it was approved by the state Senate, but he said it was “gutted” by the state Assembly’s Public Safety Committee and never made it to the Assembly floor.
So Wiener and other backers of the bill worked with law enforcement stakeholders and others to address concerns about it, including by removing some synthetic substances including LSD and MDMA from the list of drugs to be decriminalized.
Wiener introduced the updated version of the legislation in December. It would not legalize psychedelics; there would still be penalties for their sale. The bill is now headed to the Senate floor. If approved there, it will go to the Assembly, where Wiener said “it’s not guaranteed to pass, but we have a path to pass” the new version of the legislation.
Yet local dispensary owners and employees are frequently busted for selling mushrooms, psilocybin products and other illicit substances.
In April 2022, the L.A. County Sheriff’s Department tweeted that during the previous half-year, it had made 277 arrests and seized “approximately 4,000 pounds of marijuana, 3,300 pounds of marihuana edibles, 29 pounds of mushrooms and 1,000 pounds of fentanyl” from “illegal marijuana dispensaries” in the county’s unincorporated areas.
Delix Therapeutics starts clinical trial of non-hallucinatory psychedelic drug based on UC Davis research

Delix Therapeutics
Delix Therapeutics, a neuroscience company based in Boston Massachusetts, has received approval for a Phase I clinical trial examining its novel non-hallucinogenic psychoplastogen drug DLX-001.
The company announced the regulatory approval for the study on Tuesday and plans to enroll 100 healthy volunteers in the trial at the Center for Human Drug Research in the Netherlands.
The main objectives of the trial will be to assess the compound’s safety, pharmacokinetics, psychometric functions, synaptic plasticity and markers of brain activity. The company has not specified when the trial will begin.
Psychoplastogens are a class of compounds that have the potential to promote neuroplasticity in the brain as many psychedelics do with similar properties but are specifically designed to enhance brain function without inducing psychoactive effects or altered consciousness in users.
DLX-001 is the first approved psychoplastogen from Delix’s platform, based on the work of the company’s co-founder David E. Olson.
His research at the University of California, Davis, led to the discovery of psychoplastogens with significant therapeutic potential in preclinical models.
UC Davis established an Institute for Psychedelics and Neurotherapeutics in February with the intention of advancing safe and effective treatments for diseases such as depression, Parkinson’s, PTSD, substance use disorders and Alzheimer’s.





