In this week’s psychedelic news, studies suggest LSD and psilocybin could treat OCD, while Novartis halts a depression study. California pushes the TREAT California Act to further psychedelic research, and Cybin Inc. moves to acquire UK-based Small Pharma. Let’s dive in.
Psychedelic Studies and Trials
Psychedelics Unearth OCD Solutions?
Up first in our Psychedelic news roundup we spotlight a recent survey pointing to a potential indication for psychedelics. Obsessive-compulsive disorder (OCD) affects roughly 2% of people and is indiscriminate when it comes to age. Its hallmarks are intense intrusive thoughts, anxiety, and involuntary behavior, often sidelining relationships and daily activities. Treatment usually combines cognitive-behavioural therapy (CBT) with antidepressants. However, results are often slow; notably, up to 40% of patients might not find relief.
Recently, there’s been a renewed interest in a potential treatment approach dating back to the 1970s—the proposed treatment included psychedelic compounds LSD and psilocybin.
To substantiate the claims of using psychedelics to treat specific indications such as OCD, researchers need first to gather preliminary data. They can begin organizing randomized, double-blind studies if the data shows potential efficacy.
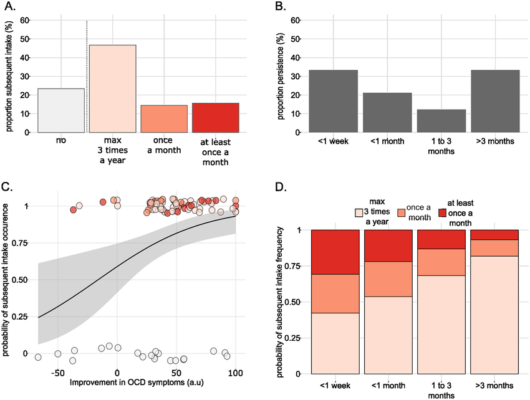
To gather an initial data set, researchers in Paris conducted a recent survey that delved into past experiences of individuals who used these substances, seeking insights on any potential symptom relief after consuming LSD or psilocybin. The longevity of these effects and any influencing factors were also under scrutiny. In a landscape lacking concrete clinical data, patient experiences and anecdotal evidence become paramount in gauging the therapeutic promise of psychedelics and steering future studies.
Anne Buot, one of the fourteen authors of this survey, shared that they gathered data from 174 individuals showing OCD symptoms and had consumed psychedelics. This questionnaire covered their mental well-being, treatments, socio-demographics, and specifics about their psychedelic use—like dosage, context, and subsequent experiences.
Feedback revealed reduced obsessive thinking, diminished ritualistic actions, less anxiety, and increased acceptance of their OCD condition. Luc Mallet, another one of the authors, noted an uplifting detail: 30% felt these beneficial effects lingered for over three months. A noteworthy observation was the direct relationship between the psychedelic dose and the depth and enjoyment of the experience.
To truly harness the promise of emerging treatments like these and set best-use standards, there’s a dual need: intensify stringent clinical research and delve into the biological workings that give psychedelics their enduring impact. The prevailing hypothesis? These substances might boost brain flexibility by fostering synaptic reshaping.
Novartis Terminates Phase II Ketamine-Like Depression Study
Across the pond in psychedelic news, Swiss pharmaceutical giant Novartis has just announced the termination of a Phase II study focused on a potential treatment for severe depression linked to suicidal thoughts. This move was driven by a comprehensive analysis of the data’s benefits and risks and made public in an October 6th update on the official federal clinical trials database. The study involved a close examination of MIJ821, an novel psychedelic intravenous solution, pitted against a placebo to determine its efficacy in alleviating depressive symptoms. Spanning one year, from July 2021 to July 2022, this trial included almost 200 participants. It’s worth noting that Novartis obtained this potential therapeutic asset following its significant 2020 acquisition of Cadent Therapeutics, for which they paid an upfront sum of $210 million.
All is not lost for MIJ821; according to the most recent update on clinicaltrials.gov, there’s an ongoing Phase II study involving MIJ821, administered as a subcutaneous injection, that is actively seeking participants diagnosed with depression unresponsive to conventional treatments.
Previously, Novartis conducted trials where they evaluated different dosages of MIJ821 in comparison with both a placebo and ketamine. They’ve described MIJ821 as having a mechanism similar to ketamine but with reduced potential for dissociative side effects.
Halucenex Phase II Psilocybin for PTSD Study Reveals Promising Results
Yesterday’s psychedelic news spotlighted exciting developments! Halucenex, a subsidiary of Melodiol Global Health, has reported promising results from their Phase II trial which tested the effects of psilocybin on severe cases of PTSD. The preliminary data suggests that their psilocybin-based product might be a groundbreaking solution for those grappling with the harrowing effects of PTSD. Out of the five individuals in the trial, an astonishing four reported significant relief after just two doses of the Lucenex solution.
Participants tested experienced a remarkable 50% reduction in anxiety and a 57% decrease in depression over the course of 44 days. ME1 has expressed considerable enthusiasm about these results, especially considering conventional treatments typically demonstrate efficacy only 20-30% of the time. Notably, three of the five participants, who initially presented severe symptoms, exhibited marked improvements by day 44. This could represent a significant stride in mental health treatment.
Real-World Psilocybin Study Reveals Significant Mental Health Benefits
The most extensive study known examining real-world psilocybin consumption revealed that individuals noted lasting decreases in feelings of anxiety, melancholy, and alcohol overuse. Additionally, they witnessed improvements in mental adaptability, emotional control, spiritual wellness, and outgoingness while experiencing a decline in tendencies toward neuroticism and feelings of exhaustion.

This research was shared in the “Frontiers in Psychiatry” journal in September, following a two-year data collection from individuals intending to consume psilocybin, primarily in the form of dried mushrooms, for self-discovery reasons.
Get more info from our full-length article here.
California: How Many Signatures Will It Take?
Californians believe it is their constitutional right to conduct more research using psychedelic substances such as MDMA, LSD, psilocybin, and ketamine, and there is a campaign to allocate $5 billion over the next few decades towards these efforts.
Last month, the attorney general’s office publicized the measure’s formal title and summary. To ensure that the potential constitutional change is included in the upcoming year’s vote, the campaign has to gather a minimum of 874,641 legitimate signatures from enrolled voters.
The campaign is now collecting those signatures.
Known as the TREAT California Act, it wouldn’t directly alter the legal standing of any substances. Therefore, it has little to do with the recent buzz around Bill 58 and the legalization of personal use. Instead, it proposes the creation of a state entity named the Treatment, Research, Education, Access and Therapies (TREAT) Institute. They aim to pinpoint and promote avenues for scientific exploration and progress in the medicinal uses of psychedelics.
The Legislative Analyst’s Office (LAO) in California shared its proposal assessment last month, projecting a cost of about $6.6 billion to the state over three decades. However, as all recipients of funding from the TREAT Institute, including businesses and universities, would enter into intellectual property arrangements, the state might recuperate a portion of this expenditure by capitalizing on novel psychedelic research outcomes.
All Votes Are In: Cybin to Aquire Small Pharma
In a significant psychedelic news development from London this week, Small Pharma, a company focused on leveraging psychedelics for short-term mental health therapies, announced that their shareholders have overwhelmingly approved a buyout by Cybin Inc. The acquisition plan, first made public in August, received near-unanimous support in the shareholder vote. Moreover, all other proposals discussed during the meeting were also approved. Not coincidentally, Cybin’s shareholders convened the same day and agreed unanimously with the acquisition.
The TSX Venture Exchange (TSXV) has provisionally approved the deal involving Small Pharma. All eyes are now on the British Columbia Supreme Court, which is expected to give its verdict on October 17. Should all conditions be fulfilled, including the definitive approval from TSXV, the deal will be sealed by October 23. Small Pharma will delist from the TSXV and cease trading on the OTCQB Venture Market.
For those unfamiliar with Small Pharma, it’s a notable biotech firm specializing in short-duration psychedelic therapies for mental health. The company has clinical assets based on N,N-Dimethyltryptamine (“DMT”), including SPL026 and SPL028. They’ve been recognized by the U.K.’s Medicines and Healthcare products Regulatory Agency (MHRA), receiving an Innovation Passport for SPL026.