
The psychedelic renaissance movement was ignited by the idea that classical psychedelics like psilocybin, MDMA, LSD and DMT are potentially more effective in treating mental health conditions than traditional medications such as SSRIs. Now, it seems like these treatments can also be used in tandem in order to increase efficacy in battling depression.
In April 2023, Small Pharma had published the results of their Phase II trial using their proprietary DMT to treat Major Depressive Disorder. The results were quite positive with two-thirds of trial patients achieving sustained remission six-months after their treatment. Of the 25 participants, 14 had entered remission in the first three months, and of those remitters, 64% maintained their remission for the entire six months.
“This data indicates that SPL026 can elicit a fast-acting antidepressant response that appears to be enduring in several cases. Recent neuroimaging and preclinical findings imply a regenerative action with DMT and other related serotonergic agonists,” commented Robin Carhart-Harris PhD, who is the Director of the Psychedelics Division at the Weill Institute for Neurosciences at the University of California San Francisco.
This week, Small Pharma published the results of the company’s Phase Ib study investigating the interaction between selective serotonin reuptake inhibitors (SSRIs) and their proprietary version of DMT (SPL026). Although studies had confirmed that combining SSRIs with psilocybin is safe though it attenuates the psychedelic effects, it was important to test safety and tolerability for patients undergoing the DMT treatment. Why? Well, requiring patients to withdraw from SSRIs before their DMT session can be a disruptive experience and can prevent patient access to this treatment. Co-administration of SPL026 with SSRIs can broaden access and streamline the clinical development pathway.
The results were quite positive. According to Small Pharma’s press release, SPL026 was well-tolerated by a 100% of patients, and no serious drug-related adverse events were reported. There were some mild to moderate adverse events observed in 8 of the patients in the SSRI cohort and 3 participants in the non-SSRI cohort, but most were resolved during the dosing visit. In other words, the results demonstrate safety and tolerability of combining DMT and SSRIs.
So far so good.
However, the most interesting and significant results are yet to be discussed. While the results reaffirmed the efficacy of Small Pharma’s Phase IIa study, the researchers observed that the antidepressant effects in the SSRI cohort were of greater magnitude, suggesting that combining SPL026 with SSRIs may potentially enhance the efficacy of DMT therapy for patients suffering from MDD.
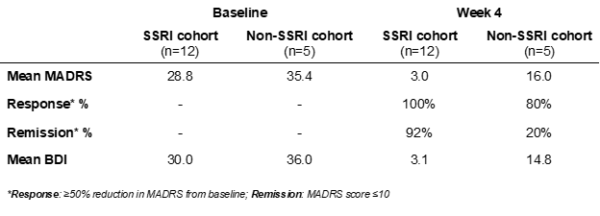
“Our primary goal in conducting this Phase Ib study was to understand if SPL026 could be safely administered in conjunction with SSRIs to assess whether patients would need to be withdrawn from their SSRI medication in future trials,” commented Dr. Carol Routledge, Chief Medical and Scientific Officer of Small Pharma. “While we were very pleased that the study demonstrated that patients may not need to be withdrawn, we were not expecting to see such a marked difference in efficacy when administering SPL026 in combination with SSRIs compared to SPL026 alone. The potentially enhanced efficacy effect of a DMT-based treatment when administered with SSRIs could lead to greater therapeutic benefit for patients, and a compelling argument for positioning it earlier in the treatment pathway. This was a small study, but the findings are both interesting and encouraging and warrant further exploration.”
Although the results are quite encouraging, it’s important to emphasize that this was a small Phase 1B study involving 171 patients with moderate to severe MDD, and the study must be replicated on a larger scale in order to validate the findings.





