Welcome to another week in the exciting world of psychedelic news. This week witnessed a wave of US states dwelling on psychedelic policies to be included in voting agendas, while cutting-edge DMT research is being subsidized for mental health studies
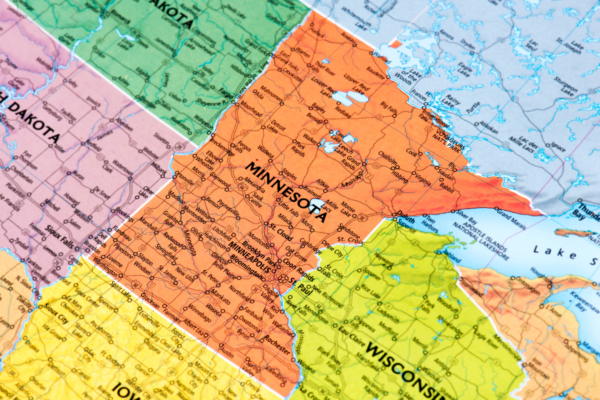
Minnesota joins list of recent states with new psilocybin legislation
As with Virginia, Indiana, Hawaii, and many others, Minnesota is entertaining the idea of psychedelic policy reform. With a focus on medical research, lawmakers are proposing the creation of a designated ‘Psychedelic Medicine Task Force’ which would investigate substances such as psilocybin, ibogaine, and MDMA for therapeutic potential.
The House bill is being sponsored by Rep. Andy Smith (D), while the Senate companion is being carried by Sen. Kelly Morrison (D). According to Representative Smith, “ This task force would establish a Psychedelic Medicine Task Force to advise the Minnesota State Legislature on the legal, medical, and policy issues associated with the legalization of psychedelic medicine in the state.”
The Bipartisan Bill further specifies that the 23-member task force be comprised of the Governor, Health Commissioner, 2 leaders each from the House and Senate. It also would include 2 tribal representatives, and various experts in fields such as public health policy, substance misuse treatment, military veterans, and others.
“Substances covered by this legislation were listed as: 3,4-methylenedioxymethamphetamine (MDMA), psilocybin, mescaline, LSD, bufotenine, DMT, 5-MeO-DMT, 2C-B, ibogaine, salvinorin A and ketamine.” In terms of a timeline for any next steps (if the bill passes), it says, “The task force shall submit two reports to the chairs and ranking minority members of the legislative committees with jurisdiction over health and human services that detail the task force’s findings regarding the legalization of psychedelic medicine in the state, including the comprehensive plan developed under subdivision. The first report must be submitted by February 1, 2024, and the second report must be submitted by January 1, 2025.”
Utah takes a slightly different approach with legislator’s newest psychedelic proposal
Utah Sen. Luz Escamilla has submitted a bill that would legalize psilocybin for medical use, following the framework of the state-run distribution program already in place for medical cannabis. This plan would enable patients to gain access to these useful medicines through a secure and regulated system.
The proposal would create initial research programs with up to 5,000 patients set to enroll. As part of the plan, two psilocybin producers will be appointed and regulated by local state laws. Patients would need to be at least 21 years old and would then try psilocybin assisted by a licensed professional. Should it prove effective, they could potentially receive a formal prescription. A separate section of the bill would also authorize psilocybin for end of life/hospice care.
This newest development comes after a recent press conference at the state capitol where several speakers (including both patients and researchers), agreed that the evidence surrounding psilocybin’s therapeutic potential has become “impossible to ignore”.
The bill was initially brought to the State Senate last week and has since been sent to the Senate Health and Human Services Committee.
The success of cannabis legalization due to the hard work and advocacy of citizens in Minnesota and Canada serves as a model for those hoping that psychedelic policy changes will follow suit.
Psychedelic reform not so agreed upon in Washington
Last month, a bill was filed by bipartisan lawmakers that would authorize the legalization of psilocybin for adults 21 and older. The expansive “Psilocybin Services Wellness and Opportunity Act” proposed by Senator Jesse Salomon (D) had 20 cosponsors in total, all supporting an effort to permit access to this psychedelic compound in a safe, regulated setting.
SB 5263 would have put Washington on a similar path as its southern neighbor Oregon. Adults 21 and older would have been able to legally use psilocybin, with the support of a trained facilitator. Regulators would have begun accepting license applications for product manufacturers, service centers and product testing labs by September 2025.
However, the Senate Labor and Commerce Committee voted to remove provisions invoking outright legality. This leaves the bill only focusing on state sponsored research related to psilocybin’s therapeutic potential as it would, “provide advice and recommendations on developing a comprehensive regulatory framework for access to regulated psilocybin.”
Advocates of the original bill claim, “The replacement bill removes the core sections of SB 5263, transforming it from a supported adult-use bill to a policy analysis bill. The substituted bill approved by Labor and Commerce would merely create a task force and a state psilocybin board to research the creation of a regulated psilocybin program.”. Similarly, a senior fellow and project lead on the Project on Psychedelics Law and Regulation (POPLAR) at Harvard Law School, said the bill “basically kicks the can down the road.”
More studies being funded and approved, this time for DMT
Clinical-stage biotechnology company, Beckley Psytech, has received FDA Investigational New Drug (IND) approval for study of ‘BPL-003’- a novel synthetic formulation of 5-MeO-DMT (Mebufotenin).
In the third quarter of 2023, BPL-003 will commence a milestone Phase IIb study to assess its safety and efficacy for individuals with treatment resistant depression (TRD). This is an unprecedented action on part of FDA, as this will be the first NDI granted for a Phase IIB exploration involving short-acting psychedelic treatments pertaining to 5-MEO-DMT. The clinical trial spans 7 countries and 40 sites, promising initial results by 2024.
Commenting on the IND approval, Cosmo Feilding Mellen, Chief Executive Officer at Beckley Psytech, said, “This is a critical step towards our mission of delivering safe, effective and licensed psychedelic treatments to patients in need, and is also a significant milestone in the evolution of our clinical pipeline. It’s a privilege to be given the FDA’s first IND for a Phase IIb study of 5-MeO-DMT and it provides firm validation of the positive preclinical and Phase I data we have generated so far with BPL-003. This is an important step not only for Beckley Psytech, but also for the whole field of psychedelic drug development and, most importantly, for patients who are in urgent need of more effective treatments for mental health conditions such as depression.”
Beckley Psytech’s Phase IIb randomized, dose-finding study will evaluate the effects of a single medium or high dose of BPL-003 against a sub-perceptual dose in patients with moderate to severe TRD who are not taking concomitant antidepressants. It will be ‘quadruply masked’, which is an intense way of saying that the patient, investigator, attendant, and outcomes assessor will all be blinded to the dose allocation of the subject to reduce expectancy bias.
Efficacy will be assessed using the Montgomery-Asberg Depression Rating Scale (MADRS) throughout the trials.
More DMT News…
Based out of the United Kingdom, Small Pharma is also conducting a study of DMT and its potential for treating major depressive disorder.
Small Pharma specializes in short-duration psychedelic-assisted therapies that mirror those of Beckley Psytech’s for mental health conditions. The company recently concluded a Phase IIa clinical trial demonstrating an “significant and clinically relevant reduction in depression symptoms”.
“SPL026 (intravenous DMT) with supportive therapy was shown to have a significant antidepressant effect that was rapid and durable, with a remission rate of 57% at three months following a single dose of SPL026,” said Dr. Carol Routledge, Small Pharma’s Chief Medical and Scientific officer.
Despite not getting the same level of attention as other psychedelics, many in the research community regard DMT highly due to its brief duration of effect. This makes it *potentially* a more effective clinical alternative than other psychedelic compounds that necessitate longer treatment appointments.
It should be noted that researchers also found, following analysis of key secondary endpoints, that there was a rapid onset of antidepressant effects one week post-dose, though no apparent antidepressant effect was observed between a one- and two-dose regimen of intravenous DMT. No abnormal effects were reported to any subjects’ health or behavior during dosing or the experience.
Small Pharma CEO George Tziras also spoke to the potential impact of the trial’s results: “Our goal is to develop proprietary, scalable and reimbursable short-duration psychedelics with supportive therapy to address this need. I am delighted with our top-line results, which demonstrate proof-of-concept for SPL026 and provide encouraging support for our broader portfolio. I want to thank each patient who took part in this trial, as well as their families, the trial investigators, the employees of the trial sites and everyone who has supported the successful completion of this study.”
The study is set for further review in upcoming scientific conferences, along with formal peer review processes prior to publication in any scientific journals.
Activist Decides to Open Shroom Store in Vancouver
Dana Larsen, well-recognized cannabis rights activist, has plans to eventually open a brick and mortar ‘compassion club’ for heroin addicts in Vancouver. However, he’s initially taking on the challenge of providing Vancouver citizens a reputable supply of psilocybin mushrooms, even as they remain federally illegal in Canada.
The Vancouver advocate founded a community drug-testing service called Get Your Drugs Tested in 2019, which offers a place for drug users to get their substances checked for harmful contaminants, seven days a week, free of charge.
“We’ve tested over 40,000 substances now,” he remarks, adding that all of the results are on a searchable database on the website. “We’re doing like two-thirds of all the drug testing in the province from our one location at 880 East Hastings.”
Larsen also sells DMT and peyote, which are derived from plants with psychoactive qualities, as well as coca leaf tea — an herbal tea that is derived from the same plant that cocaine comes from.
Kratom, another item on the menu, comes from a tree in southeast Asia and is used as a substitute for street opiates and fentanyl. While it is not in the Controlled Substances Act, it is not to be sold for “human consumption.”
“I hope that this is the beginning of more stores like this in Vancouver,” he says. “This is part of an effort to end the war on drugs by trying to do civil disobedience.”
Learn more about the Psychedelic Gray Market:
An Updated (Living) List of North American Psychedelic Dispensaries
.jpg)






